A Novel Mechanism of Cell Proliferation Discovered In HUN-REN IEM Could Play a Role in The Formation of Human Gliomas
A novel molecular mechanism regulating glial cell proliferation in the central nervous system was discovered by the HUN-REN Institute of Experimental Medicine’s (IEM) Molecular Cell Metabolism Research Group led by Dr. Balázs Gereben. First authored by Dr. Petra Mohácsik and published recently by the Journal of Biological Chemistry, this novel mechanism may also play a role in the oncogenesis of human gliomas.
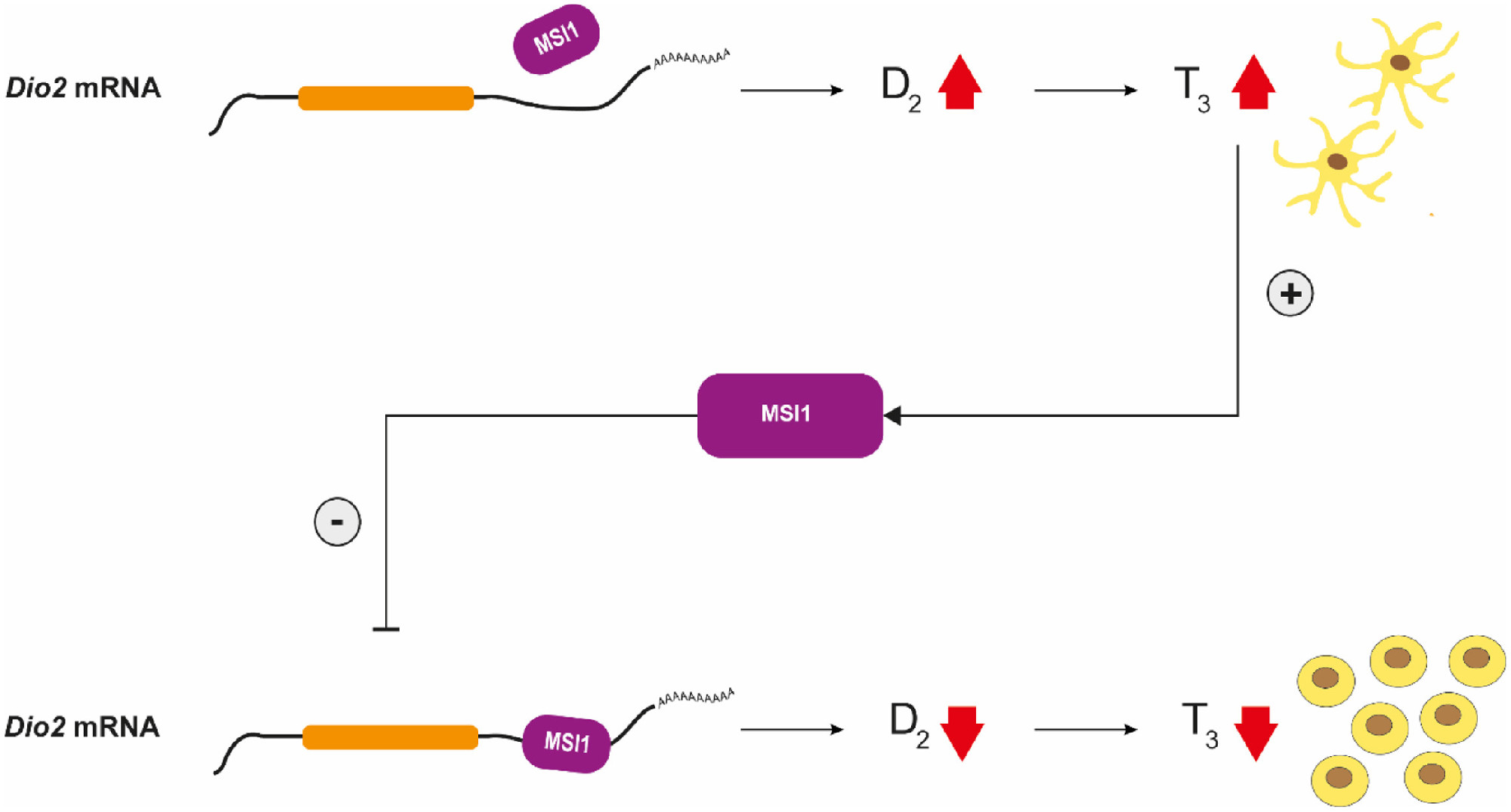
Beyond neurons, glial cells represent the other major cell-type of the nervous system. Astrocytes, representing a crucial glial subtype and the most abundant cell-type in the brain, are present in all brain regions and play a fundamental role to provide appropriate conditions for proper neuronal function. Tight regulation of glial cell functions is ensured by numerous factors, including hormonal pathways. Thyroid hormone (TH) is a critical regulator of cellular proliferation and function. While circulating TH level is relatively stable in the bloodstream, tissue TH action fluctuates according to cell-type specific mechanisms. The crucial step of TH action is the conversion of the prohormone thyroxin (T4) to the activated form, T3 that can bind the thyroid hormone receptor. This reaction is catalyzed in the brain by the glial type 2 deiodinase (D2) selenoenzyme.
The researchers of HUN-REN IEM identified a novel molecular mechanism that determines T3 availability in the glial compartment of the brain via the regulation of the D2 enzyme. The D2 mRNA has an unusually long 3’ untranslated region (3’UTR), a region that is not encoding the D2 protein itself but provides a target for regulatory factors. Using molecular biological techniques, they proved that Musashi-1 (MSI1), a highly conserved RNA-binding cell cycle regulator protein directly binds this RNA region. They found in cell cultures including human glioma cells that MSI1 downregulates D2 activity and consequently its capacity to generate T3. This finding demonstrated a tight molecular link between MSI1, a proliferation promoting factor and T3, that support cell differentiation.
Their in vivo studies in the mouse brain proved the co-existence of MSI1 and D2 in glial elements that provided the morphological basis of significance while direct evidence on functionality of the MSi1-D2 pathway was obtained in an MSI1-lacking transgenic mouse model.
Mature astrocytes are not dividing in the adult brain, however, under specific circumstances (e.g. focal ischemia, traumatic brain injury or brain tumors) they resume stem cell-like properties and the ability to proliferate. Astrocytomas and glioblastomas are intensely proliferating and invasive malignant tumor types of the brain that originate from glial cells. Therefore, it is important, that the researchers also found that modulation of the MSI1-D2 pathway by performing an Msi1 knock-down induced increase of D2 activity slowed down astrocyte proliferation by 56% that was directly dependent on thyroid hormone. This proved the role of MSI1-D2-T3 pathway in the regulation of astrocytic cell cycle.
The findings were achieved in collaboration with the groups of Dr. Csaba Fekete (HUN-REN IEM) and Dr. AC Bianco (University of Chicago, IL: USA) and proved that the MSI1-D2-T3 pathway is a novel molecular regulatory mechanism of astrocyte proliferation with the potential to impact the pathogenesis of human glioblastomas. The project was supported by the US National Institute of Health (NIDDK R01DK058538), The National Laboratory of Translational Neuroscience and the National Research, Development and Innovation Office of Hungary.