HUN-REN BRC Researchers Develop Laser-guided Microrobots for Cell-capturing
Researchers at the HUN-REN Biological Research Centre, Szeged, have developed tiny tools to capture individual cells. According to their study published in the journal Advanced Materials, key innovations of using flexible microrobots is that they do not require any treatment of the cells to grab them and also allow the cells to be released after examination, enabling more efficient investigations than ever before.
Single-cell investigation methods such as single-cell genetics, proteomics, or imaging-based morphological classification have risen to the forefront of biological research in the last decade. These methods require precisely controlled physical manipulation of individual cells on the microscopic scale. This manipulation at the single-cell level means their transportation and rotation in a controlled manner, for which several methods have been developed over the last decades. These cutting-edge methods use active movable microtools such as microgrippers similar in size to the cells, complex electrophoretic systems that use high-frequency electric fields to move the cells, or optothermal traps that create liquid flow through localised laser heating. The technique of optical tweezers fits into this category, being one of the most efficient single-cell manipulation methods and was awarded a Nobel prize in 2018. Optical tweezers consist of a highly focused laser beam, in the focus of which micrometre-sized objects, including cells, can be trapped. A more robust and effective way of taking hold of cells is their indirect trapping using intermediate objects as handles, which provide considerably larger tapping force and cause less photodamage to sensitive cells. These handles, which can take the form of a simple microsphere or a more complex structure, are strongly bound to the cells, requiring some sort of physico- or biochemical treatment of both parties. Besides the inevitable advantages of such handles, the treatment itself, and the fact that they cannot be removed from the cells, are often disadvantageous.
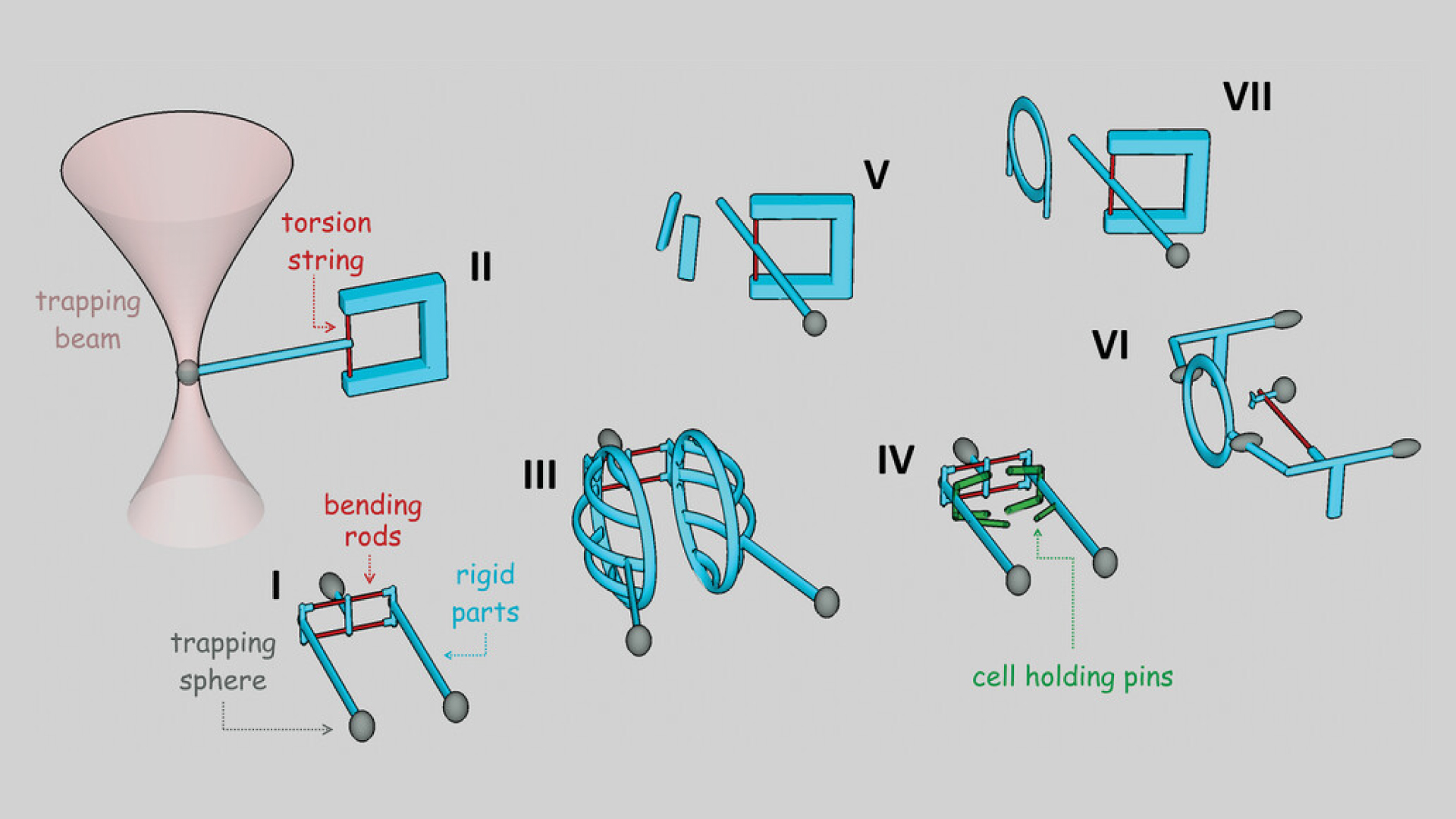
A solution for this problem was offered by the group of researchers led by Lóránd Kelemen at the Institute of Biophysics at the HUN-REN Biological Research Centre, Szeged, in cooperation with their Slovakian partners, who developed a novel family of deformable single-cell manipulation microtools that can be transiently attached to cells. The key feature of the structures is their elasticity, which enables them to hold cells without the need for any kind of treatment on them or on the cell. These tools act as tiny robots that can grab, hold still, rotate, move, and release the cells under study. The microtools are operated using multifocus optical tweezers and can undergo a very high degree of deformation in order to accommodate cells. The deformable parts of the structures are as thin as 300 nanometres, which is necessary for the optical tweezers to bend them. The microstructures are also manufactured by laser, using an additive microfabrication technique, called two-photon polymerisation. This methodology is essential to produce microrobots, since it is one of the few techniques capable of preparing arbitrarily shaped 3D polymer objects with feature sizes down to even 100 nanometres.
Schematic representations of the elastic, deformable microstructures used in the experiments.
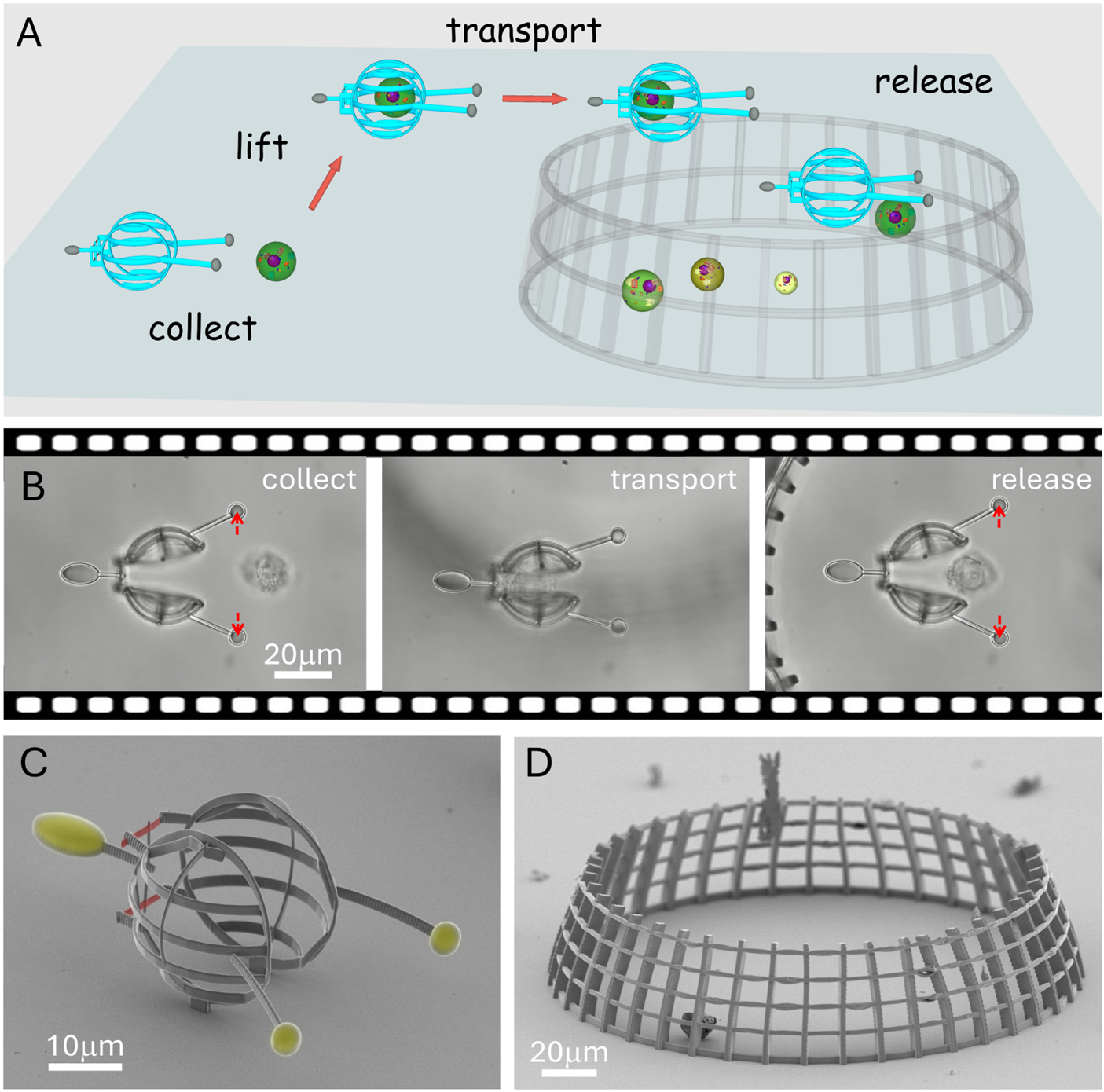
The researchers demonstrated the capability of the technique through three types of structures specifically designed for three distinct cell manipulation tasks. The first is a cell transporter designed to enclose and move a cell selected from among many surrounding cells in a microfluidic environment and transport it with practically no force applied on it. It consists of two half spheres that are about twice the size of the cell, which can be opened up with the tweezers to accommodate the cell conveniently. Its cargo can then be transported to and released at any area defined by the user. The second type is somewhat the opposite: it is designed to hold the cell firmly, allowing it to fluctuate only 50 nanometres. The purpose of this tool is to keep the cell still for imaging with the huge additional benefit of being able to rotate it to any preferred orientation under the microscope to access imaging directions at will. The potential of this microtool was demonstrated by using it to improve the optical resolution of 3D fluorescence imaging of the cell. Finally, the third member of the microrobot group is actually a pair of structures used to initiate cell-cell interaction with precise spatial and temporal control over the process. One of the structures holds a cell firmly, while the other manoeuvres a second cell to the first one with the optical tweezers and presses them together. The main advantage of this setup is that the onset of the interaction can be accurately defined in contrast to experiments where it is randomly initiated in a test tube by simply mixing the cell suspensions. The tools enable the precise observation of time evolution of how the two cells react to each other, essential to discover the details of their behaviour even at the molecular level. The researchers believe that the presented optical tweezers-based methodology using task-specific microtools will allow the investigation of single, non-adherent cells in a much more efficient way and proves that soft micro-robotics has huge potential in biological applications.
There is also a video on X showing the special movements of microrobots:
https://x.com/AdvSciNews/status/1800468454199181564