Hungarian researchers develop novel antibiotic to combat superbacteria
The WHO predicts that by 2050, the leading cause of death could be infections caused by microorganisms resistant to conventional antibiotics. To combat these, new types of compounds with specific mechanisms of action are needed. Researchers from the HUN-REN Research Centre for Natural Sciences (HUN-REN RCNS) have reported a possible promising direction in their study published in the prestigious journal Nature Communications.
It is no coincidence that superbacteria resistant to conventional antibiotics have become the focus of research: the WHO’s first comprehensive analysis in 2020 already showed that resistant bacteria caused 1.27 million deaths in 2019, and unfortunately, this number multiplies each year. One way to fight superbacteria is phage therapy, where viruses are used to kill them. Such research is being carried out, for instance, at the HUN-REN Biological Research Centre in Szeged. Another promising option is the development of antibiotics with completely new mechanisms of action.
“Bacterial microsystems are incredibly adaptive, sometimes building up an entire colony overnight. Moreover, the latest research suggests that certain bacteria secrete nanometre-sized ‘alarm globules’ upon their death, thereby passing on survival knowledge to their peers. This incredible adaptability suggests that a multifaceted approach is required to combat superbacteria,” explained Tamás Beke-Somfai, leader of the Biomolecular Self-assembly Research Group at HUN-REN RCNS.
“Even in our own bodies, we have natural peptides known as antimicrobial peptides. We have designed supramolecular structures – essentially a new antibiotic – that combine the bactericidal mechanisms of these natural peptides with the favourable biostability of non-natural compounds,” summarised Tamás Beke-Somfai. Until now, researchers have not been able to truly observe how peptides “attack,” but the Hungarian researchers have now successfully tracked how they arrange into supramolecular structures on the surface of bacterial cells using transmission and cryo-electron microscopy.
“The recordings show that the forming lamellae grow into the cell wall such as knives and penetrate the bacterium. Puncturing the cell wall causes leakage, which eventually leads to the bacterium’s death. Image analysis also revealed that surprisingly few of the lamellae penetrating the bacterial cell are sufficient to cause cell wall damage. Moreover, the tests indicate that the peptides inhibit bacterial growth even at very low concentrations,” summarised the research group leader.
The microscopic images and molecular dynamics simulations confirmed that the formation of peptide-based lamellae is based on the interaction with phosphate groups: even in the presence of simple inorganic phosphate ions, striped lamellar morphologies form where individual layers consist of two or more peptide layers connected by phosphate ions and stabilised by hydrogen bonds.
The work of the HUN-REN RCNS researchers is considered innovative in several respects: firstly, they successfully designed a peptide-based antibiotic that is activated in situ by binding specifically to bacterial cells; secondly, they are the first to gain direct visual insight into the function of bactericidal peptides.
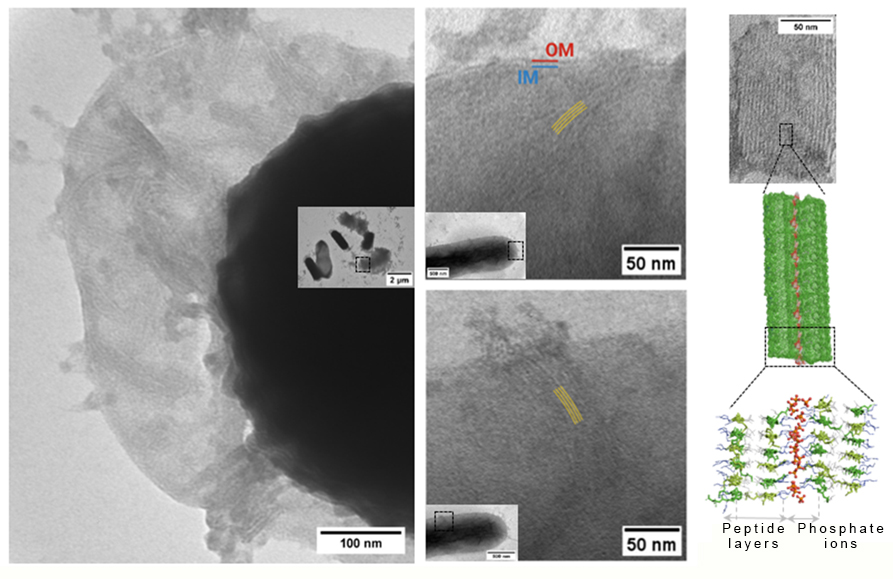
Peptide lamellae (yellow stripes) form on the surface of bacteria and disrupt the cell wall (OM and IM) as they grow into it (left). Molecular structure of peptide-based supramolecules (right).

