New Discovery by IEM Researchers Could Improve Understanding of Brain Disease Mechanisms
Microglial cells are highly sensitive to tissue injury and respond to damage with characteristic morphological and functional transformations. Despite these marked phenotypic and functional changes, microglia contribute to the regeneration of neuronal network activity, as demonstrated in acute brain slice preparations by the Laboratory of Neuroimmunology of the HUN-REN Institute of Experimental Medicine (HUN-REN IEM) led by Ádám Dénes. The study, co-authored by Péter Berki, Csaba Cserép and Zsuzsanna Környei, with the collaboration of Chinese, American and German researchers, was published in the prestigious international journal Nature Communications.
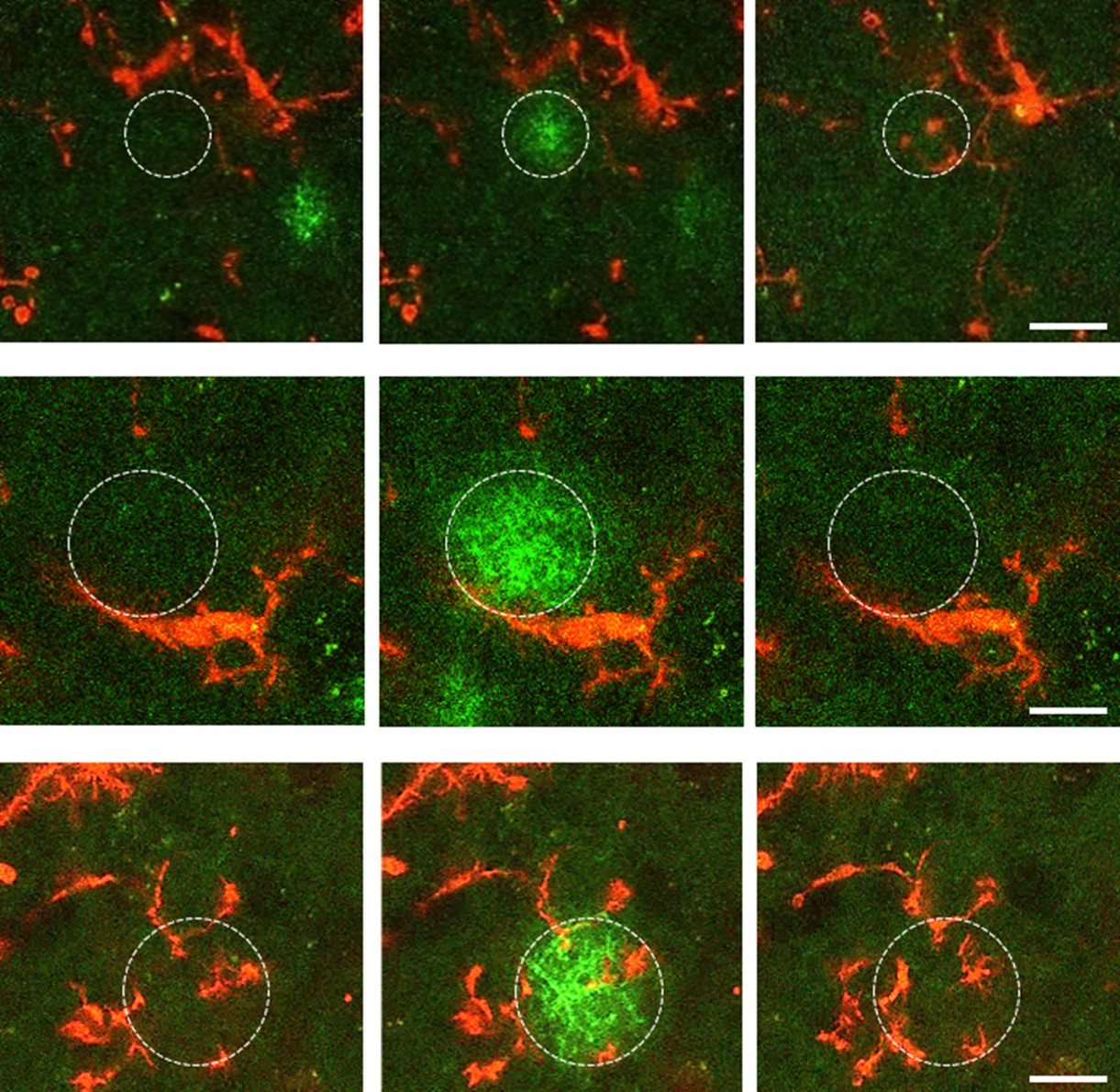
The research group, which a few years ago first described direct somatic communication between microglial cells—known as the primary immune cells and the main regulators of inflammatory processes in the brain—and neurons, has now published a paper elaborating on the impact of microglial phenotype changes on the functioning of complex neural networks. Using a versatile methodological approach, they have demonstrated that microglia in acute brain slice preparations undergo rapid and robust phenotype changes initiated immediately after slice preparation. Microglial cells retract their processes while migrating towards the surface of the slices. During this time, their connections with neurons and synapses are altered, and their cell surface receptor repertoire is also transformed. Using a fluorescent ATP sensor developed by the Chinese co-authors, immediate ATP release was observed in response to slice cutting, followed by rapid, flashing, focal ATP events.
Surprisingly, these flashing ATP events mediating injury-related responses to microglia were observed even hours after the initial 'ATP storm' had subsided. The researchers revealed that the complex response of microglia is driven in part by this dynamic ATP release due to tissue injury. Several fundamental signalling pathways are responsible for the rapid and robust changes in microglial cells, which, in parallel with the complex microglial responses, induce neuronal network remodelling required for the emergence of synchronous neuronal activity. The researchers have also demonstrated that microglial cells are involved in the formation of complex neuronal activity patterns that are crucial for learning and memory processes, not only in brain slice preparations, but also in the intact brain in vivo.
The mechanisms identified in this paper could advance the understanding of common brain disorders (e.g. traumatic brain injury, stroke, ischaemia) and also chronic neurodegenerative diseases such as Alzheimer's or Parkinson's disease, dementia and epilepsy.
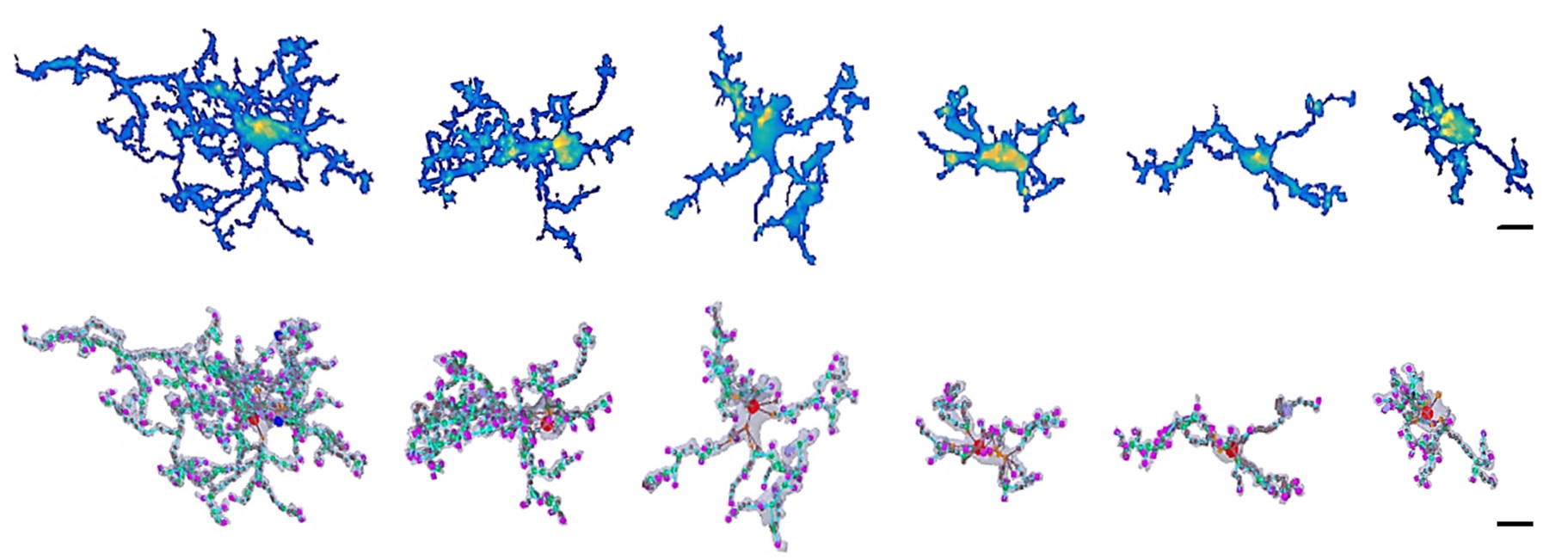
Figure 1. Microglial cells undergo dramatic morphological (and functional) changes during a 5-hour period following brain slice preparation.