New Molecular Principle Discovered by HUN-REN IEM Researchers Brings Us Closer to a Better Understanding of the Effects of Cannabis
A recent study conducted by the HUN-REN Institute of Experimental Medicine (HUN-REN IEM) contributes to our understanding of the molecular-level phenomenon of tolerance that occurs with regular cannabis use. The methodology developed by István Katona and his colleagues enables the direct investigation of the nanoscale molecular organisation that underlies the physiological processes in synapses. The new approach revealed how the precise spatial positioning of cannabinoid receptors regulates synaptic efficiency. The psychoactive substance in cannabis alters the nanoscale arrangement of cannabinoid receptors, thereby influencing the strength of the synapses. This research, with PhD student Benjámin Barti as the lead author, was highlighted on the cover of the May issue of Science Advances, a highly prestigious scientific journal.
Our brain's approximately 86 billion neurons communicate with each other through about 100 trillion synapses. The efficiency of synaptic function dynamically changes in space and time, which is extremely important in the coding processes of our nervous system. According to the traditional principle of synaptic transmission, one neuron sends neurotransmitter molecules to another neuron, thereby influencing the function of the receiving neuron. Feedback mechanisms, which allow the receiving neuron to send feedback to the first neuron, are vital in determining the strength of synapses. Interestingly, the most common synaptic feedback mechanism was discovered unexpectedly, with HUN-REN IEM researchers playing a significant role in this discovery. Their first paper on this topic was published 25 years ago in the Journal of Neuroscience and has since become one of the most cited studies among publications originated in Hungarian laboratories. István Katona and Beáta Sperlágh, who were young researchers in the research groups led by Tamás Freund and E. Szilveszter Vizi at the time, were the first to directly demonstrate that the receptor for the psychoactive substance of cannabis is located on nerve endings and inhibits the release of neurotransmitters. Based on their results, they already predicted in 1999 that the physiological role of cannabinoid receptors is the regulation of the feedback processes of synapses.
This hypothesis was later confirmed by various research groups. Over the past two decades, researchers from HUN-REN IEM have significantly contributed to the elucidation of the molecular and anatomical organisation of this pathway, as well as to the discovery of its physiological and pathophysiological significance. However, a fundamental question remains unanswered: What determines the strength of feedback in each synapse, or in other words, which kind of quantitative molecular principles underlie the strength of retrograde synaptic signalling?
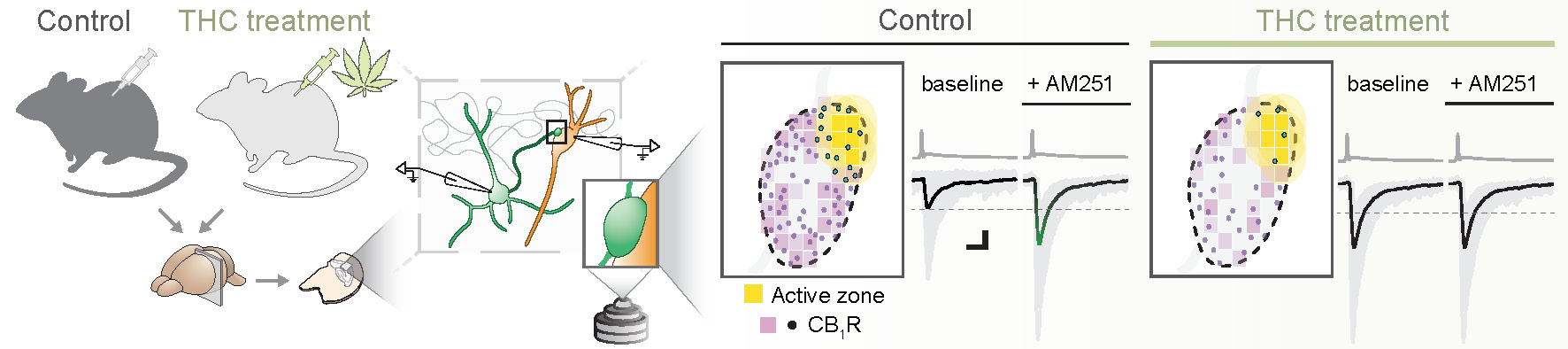
The study’s experimental design represented schematically. The researchers investigated groups of mice that were either in the control group or treated with THC. Subsequently, the brains were removed, and acute brain slices were prepared for further analysis. They conducted electrophysiological and super-resolution microscopic analyses on these slices. This analysis revealed an alteration in the nanoscale arrangement of cannabinoid receptors in the THC-treated group, which is likely to underlie the observed physiological changes.
The challenge in answering this question lies in the microscopic scale of the synapses. An average nerve ending is only 1 micrometre in diameter, and the synaptic gap between nerve cells is a mere 10 nanometres, which is one hundred thousandth of a millimetre. This makes it extremely difficult to examine the molecular organisation of synapses using traditional light microscopy methods. In 2015 and 2016, István Katona and his colleagues published a series of papers in the journals Nature Neuroscience and Nature Protocols, introducing a method based on super-resolution microscopy. This method allowed, for the first time, the measurement of the nanoscale abundance of molecules involved in synaptic signalling processes of a specific type of neuron with nanometre precision. In their latest study, they further refined this method. They developed a procedure that first measures the strength of signal transmission in the synapse connecting two nerve cells, and then quantifies the spatial arrangement of the molecules involved in signal transmission with nanometre precision using a super-resolution microscope. During their experiments, they studied a unique synaptic phenomenon known as the cannabinoid tone. According to a 2013 discovery by the group of Nobel laureate Thomas Südhof, the cannabinoid tone is absent in a type of autism with a specific genetic background, while the traditional endocannabinoid signalling pathways remain unaffected. The reason for this discrepancy remained unknown.
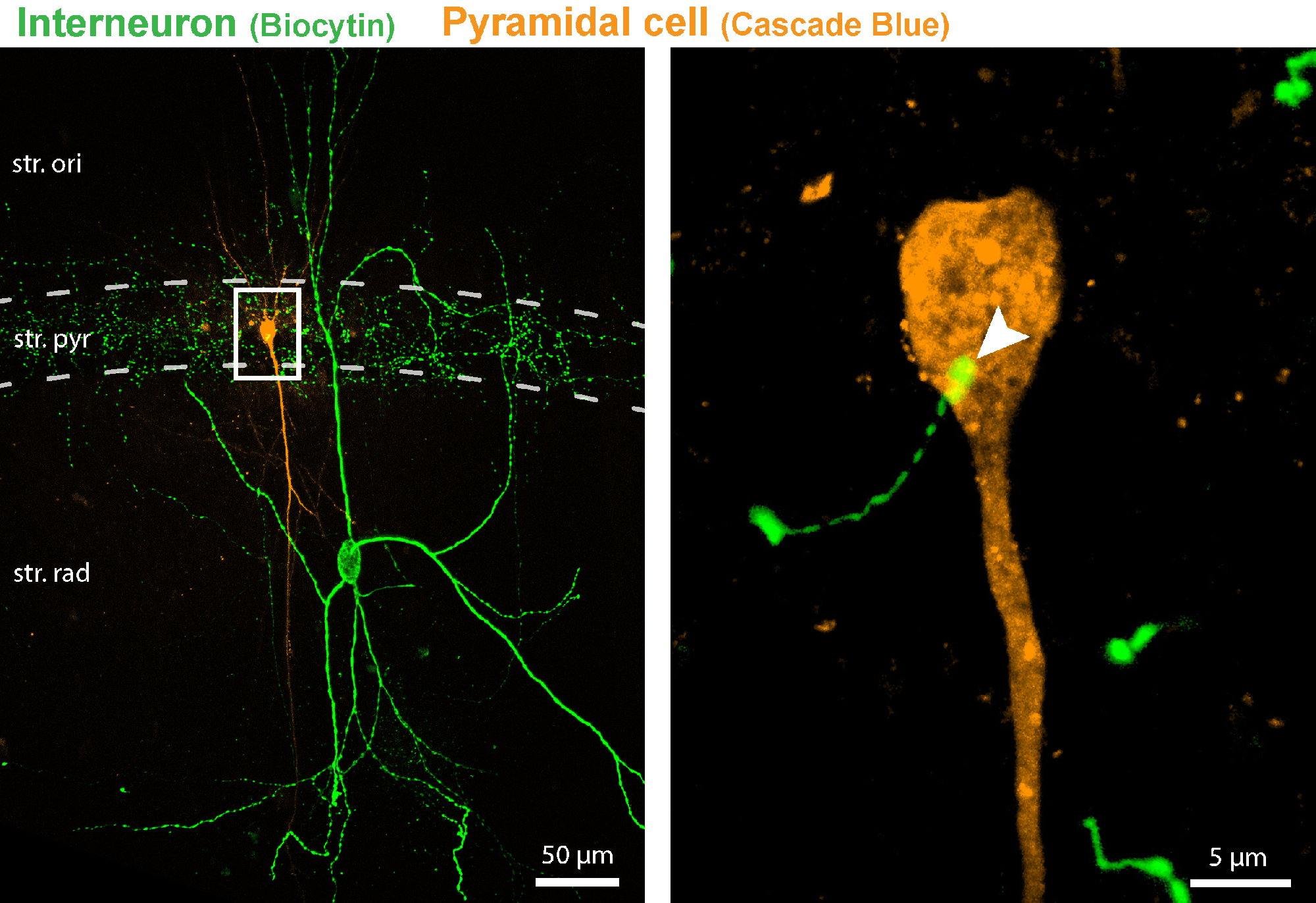
A confocal microscopic image from the study shows a pair of a presynaptic interneuron (green) and a postsynaptic pyramidal cell (orange), both labelled with fluorescent markers. The boxed region, enlarged on the right side of the image, reveals a synaptic connection between the two cells (indicated by an arrowhead).
Based on an initial surprising observation by HUN-REN IEM researchers, the two classic endocannabinoid-producing enzymes are not involved in this synaptic phenomenon, suggesting that a new molecular mechanism underlies the cannabinoid tone. The researchers then tested their hypothesis that a group of cannabinoid receptors is continuously active even in the absence of endocannabinoid signal molecules, thereby regulating the strength of synapses. Physiological and molecular measurements revealed that the number of tonically active cannabinoid receptors in the 200-nanometre environment of neurotransmitter release sites significantly influences the strength of synaptic communication between neurons. Finally, they investigated how a six-day treatment with the psychoactive ingredient of cannabis affects the nanometre-scale arrangement of cannabinoid receptors in mice. They found that the number of cannabinoid receptors in the 200-nanometre area of the neurotransmitter release site was halved. Simultaneously, the phenomenon of cannabinoid tone completely disappeared from the synapses, while the functioning of the classical endocannabinoid signalling pathway showed only a minor change. This unexpected result suggests that among the many effects of cannabis, there may be molecular changes like those observed in a genetic form of autism spectrum disorder.

