A Breakthrough Discovery in the Fight Against Superbugs Led by Hungarian Research Group
Hungarian researchers have created a unique superbug map as part of an international project, providing a detailed depiction of the global presence and spread of one of the most dangerous hospital bacteria. The research, led by the Biotechnology National Laboratory at the HUN-REN Biological Research Centre, Szeged could open a new chapter in combating antibiotic-resistant hospital infections. Using this map, the scientists are developing bacteriophage-based treatments that—as promising alternatives to ineffective antibiotics—specifically destroy the most prevalent superbugs. The quality of the research and the significance of the topic are underscored by the publication of their results in Cell, one of the world's most prestigious life science journals.

Bálint Kintses
The research groups led by Bálint Kintses and Balázs Papp have made significant progress in curbing hospital infections—a global problem—using a new approach. In cooperation with Hungary’s national epidemiological and drug regulatory authority, the National Center for Public Health and Pharmacy (NNGYK), the National Laboratory of Health Security, and numerous hospitals from five Eastern European countries - including Hungary - they have created an unparalleled map of the global occurrence of Acinetobacter baumannii, one of the most dangerous hospital pathogens, and the patterns of spread of its various strains. This information is extremely valuable for predicting outbreaks and drug development.

Balázs Papp
One Bacterial Species, Hundreds of Variants
The “superbug map” is based on a detailed analysis of bacterial genomes, providing precise information about the diversity within the studied species. Antibiotic-resistant bacteria can have hundreds of variants within a single species. This makes it challenging to use phages in therapy because each variant might require a different phage to effectively target it.
Fighting Bacteria with Good Viruses
Bacteriophages are “good” viruses that only infect and destroy bacteria, leaving human cells unharmed. This makes them ideal for fighting bacterial infections. However, phages are highly specific: within a bacterial species, a specific phage must be identified for each variant. This significantly complicates the application of effective phage therapy, especially in fast-progressing infections that do not respond to antibiotics. Once an infection has developed, there is typically only a one- to two-week window to reverse the patient's deterioration. This short timeframe makes it difficult to identify the exact bacterial variant, find an effective bacteriophage against it, produce the phage preparation for therapeutic use, and obtain regulatory approval.
Hope for Approval of Phage-Based Medications
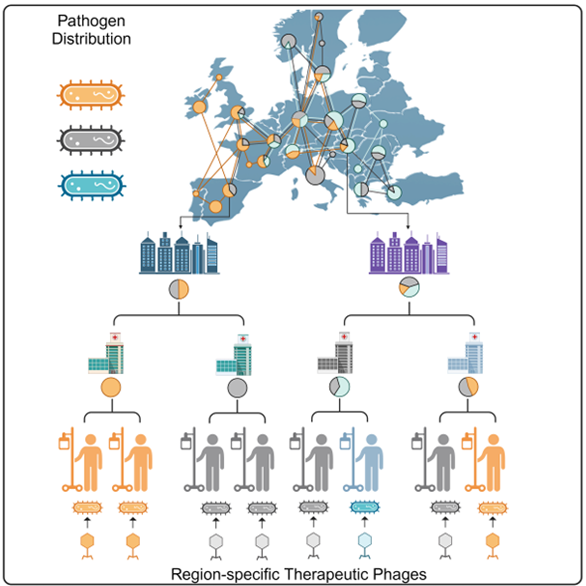
Using this unprecedented global map, the researchers discovered that although the diversity within species is vast, only a few variants dominate in any given region. If at least 8–10 different bacteriophage preparations tailored to specific regions were available, 80% of hospital infections caused by this bacterium could be treated. Animal experiments have also confirmed that effective phage combinations can be assembled against the strains occurring in the Eastern European region.
Knowing which regions are affected by the same bacterial variants infecting hospitalized patients—who are particularly vulnerable to these infections—allows for more effective clinical trials with targeted phage preparations through global collaboration. One of the main obstacles to the approval of bacteriophage therapy has been the inability to identify which patients require the same phage treatment, leading to unsuccessful clinical trials. For phage therapy to become widely accessible, regulatory guidelines require demonstrating the efficacy and safety of these preparations in an adequate number of patients. The superbug map could bring a breakthrough in this area and offers hope that phage therapy may become an integral part of the most modern tools of personalized patient care in the not-too-distant future—just as antibiotics did in the mid-20th century.
Further Information About the Research
How Was the Superbug Map Created and What Is Its Purpose?
By analyzing samples collected from hospitals across Eastern Europe, the research community precisely identified the different strains of Acinetobacter baumannii. Alongside the participating hospitals and university centers, the National Center for Public Health and Pharmacy (NNGYK) played a crucial role in sample collection and genome sequencing; their experts also combat hospital infections using modern genomic tools. By integrating data from the NNGYK and other large public databases, the researchers compared their collected data with bacterial variants found in countries on other continents, mapping geographical and temporal occurrences in a complex system. The resulting global map not only reveals which regions of the world harbor the same variants of the pathogen but also excellently characterizes the spatial and temporal spread of individual strains.
How Do Superbugs Spread?
The researchers discovered that the spread of multidrug-resistant bacteria follows a star-shaped pattern: typically starting from capital city hospitals and spreading towards regional institutions. The superbug variants spreading nationwide from high-patient-volume metropolitan hospitals change relatively slowly. This allows predictions at national, regional, or even city levels about which subtypes of superbacteria are likely to appear in hospitals in the coming years. This can enable preemptive preparation by developing effective phage cocktails against the variants most likely to emerge.
From Theory to Reality
This new methodology of superbug mapping paves the way for the widespread application of bacteriophage therapy. Although some countries already permit phage therapy under specific conditions (typically only for slowly progressing disease forms representing a small fraction of incurable infections), practical implementation remains challenging. This research offers hope that this could change in the near future. Just as a decade ago, the clinical application of stem cell therapy or gene therapy seemed like science fiction, it is conceivable that in the coming decade, widely available phage therapy will become a reality in clinical practice.
By Dr. Dóra Bokor

