A Development by Hungarian Researchers Could Give Fresh Impetus to The Fight Against Cancer
In preclinical studies conducted to date in animals, an experimental anticancer agent developed by Hungarian researchers inhibited the growth of skin and lung cancers and achieved complete tumour regression in breast cancer. The joint development by the HUN-REN Research Centre for Natural Sciences (HUN-REN RCNS) and Eötvös Loránd University (ELTE) could mark the beginning of a new era in anti-tumour therapies. Their findings were recently published in the prestigious international journal Molecular Cancer.
In their recently published study, the researchers demonstrated how they ‘tamed’ a highly toxic anticancer compound, pyrrolino-daunorubicin, by encapsulating it in a liposomal nanoformulation. The resulting compound, dubbed LiPyDau (liposomal pyrrolino-daunorubicin), showed exceptional efficacy, surprising even the researchers.
In animal studies, LiPyDau inhibited tumour growth across six distinct tumour models, including models of melanoma and lung cancer. In a hereditary breast cancer model, it induced complete tumour regression. Importantly, LiPyDau was also effective against drug-resistant tumours that show little or no response to currently used clinical agents.
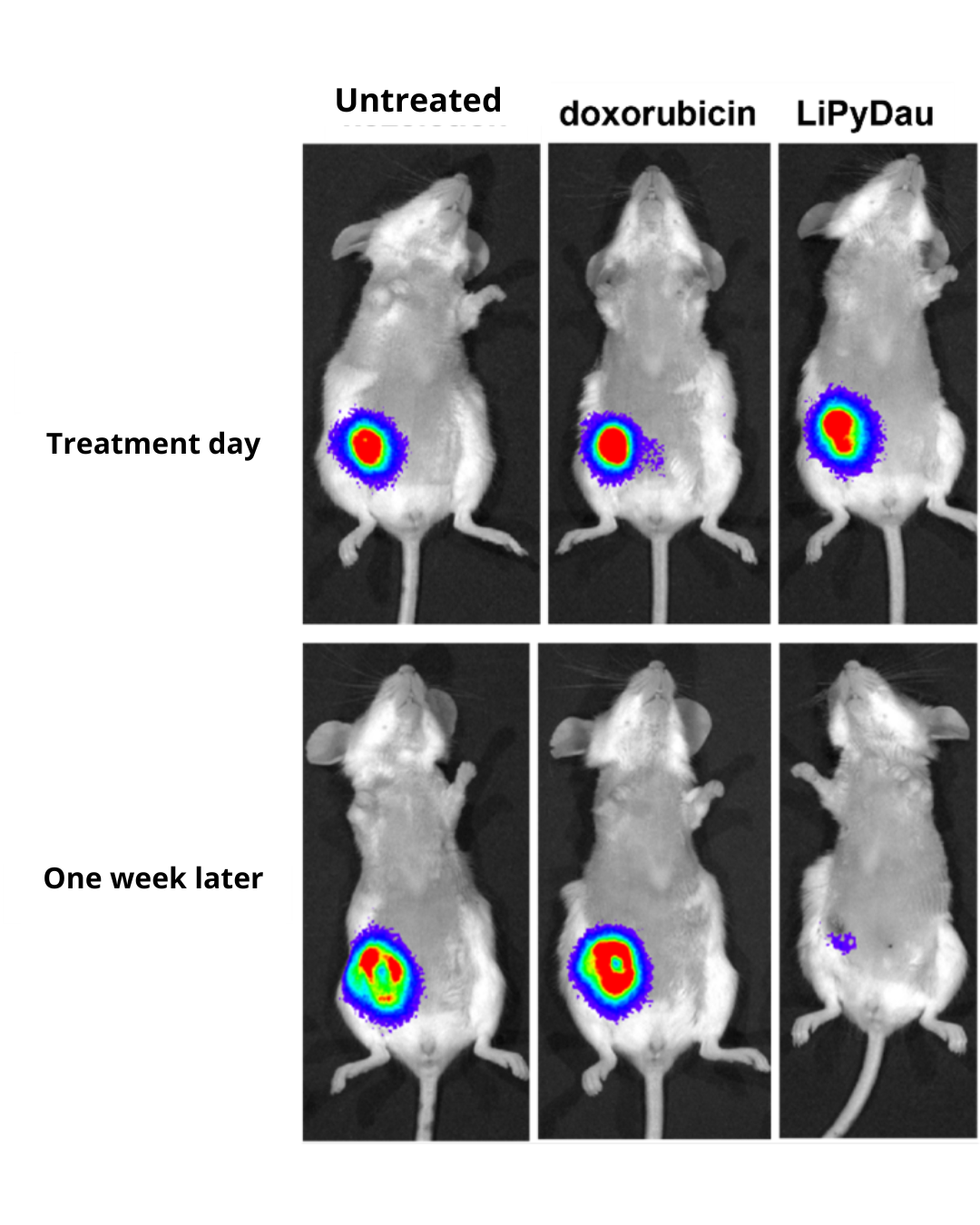
With a single treatment, LiPyDau eradicated tumours derived from 4T1 mouse mammary tumour cells, one of the most treatment-resistant murine breast cancer models, within one week. Luminescent imaging shows tumour size and location (visible here as false-colour patches on the animals’ bodies). Photo: Dr Kata Bölcskei, Department of Pharmacology and Pharmacotherapy, Medical School, University of Pécs
The fruit of a decade of research
Dr Kristóf Hegedüs, who worked on the compound’s synthesis under the supervision of Dr Gábor Mező at Eötvös Loránd University (ELTE), said that once they recognised how promising the molecule was, they began developing the techniques needed to produce it at the required quality and in sufficient quantities. ‘This opened the way to animal studies’, added the HUN-REN RCNS researcher.
The candidate compound, pyrrolino-daunorubicin, is approximately a thousand-fold more cytotoxic to cancer cells than conventional agents. However, administering it to animal models without harming healthy tissues posed a major challenge. ‘That was when the idea of a liposomal nanoformulation emerged. It would shield healthy cells from the active compound while enabling targeted accumulation in the tumour. That is how LiPyDau was born,’ said Dr Szilárd Tóth, who led the testing of LiPyDau in cell lines.
According to Dr András Füredi, one of the study’s lead authors, this is not merely an effective formulation; based on their experience and the published literature, it ranks among the most potent anti-tumour compounds ever tested in mice. ‘We have been developing cancer therapies for fifteen years, but we have never observed such a marked effect. A single dose of LiPyDau cured the experimental animals,’ the researcher emphasised.
Within HUN-REN RCNS, the research was the result of broad collaboration among multiple groups: alongside Dr Gergely Szakács’s team, the units led by Dr Zoltán Varga, Dr Pál Szabó and Dr Dávid Szüts were indispensable to the successful development and testing of LiPyDau, while Dr Csaba Magyar carried out the molecule’s in silico modelling. The research groups behind the discovery worked closely together on the project for nearly a decade.
A Hungarian success built on collaboration
Dr Gergely Szakács (HUN-REN RCNS), who led the research, emphasised the exemplary national collaboration behind the project. Partners included the National Laboratory of Pharmaceutical Research and Development, the HUN-REN Centre for Energy Research, Óbuda University, the University of Pécs, the National Institute of Oncology, the Medical University of Vienna and the University of Veterinary Medicine Vienna. The research was also supported by Kineto Lab Ltd., one of Hungary’s leading oncology development companies, with whom they are already planning the next steps towards clinical trials of LiPyDau. However, further studies are required to determine whether these promising results can be translated into clinical practice.
The aim is clinical trials
Asked when patients might benefit from LiPyDau’s efficacy and whether an experimental formulation of this kind could reach clinical practice, Attila Kigyós, founder and CEO of Kineto Lab Ltd., responded: ‘LiPyDau is by far the most effective anti-tumour compound we have tested in the company’s history. We must do everything we can to advance it to a stage where clinical trials are possible, or where it attracts the interest of a major pharmaceutical company to acquire it and develop it into an oncology product.’ He added that LiPyDau stands a strong chance of becoming a Hungarian-developed cancer medicine.

