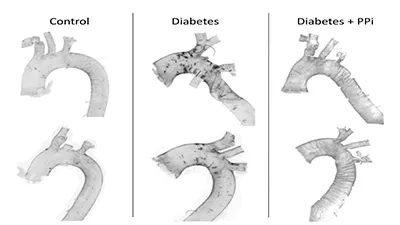
About 10% of the population has diabetes mellitus (DM), and the prevalence of diabetes keeps rising. DM accelerates atherosclerosis, peripheral arterial disease is a major complication and the leading cause of amputation among diabetics. Vascular calcification is an independent predictor of cardiovascular and overall mortalities in patients with type 2 diabetes. To examine this pathogenesis in more detail, we developed a diabetic mouse model exhibiting diabetes-associated cardiovascular pathology, such model was not available earlier. Our diabetic mice develop accelerated and increased calcification in their aortas and kidneys compared to age-matched controls, providing a unique tool to investigate diabetes-related vascular calcification. Our preliminary results show that under diabetic conditions, plasma pyrophosphate (PPi) levels decrease in our mice indicating that PPi may play an important role in the prevention of diabetes-related calcification. To directly address this pathogenesis, we proposed a PPi replacement therapy. We hypothesized that oral supplementation therapy using PPi can alleviate this
complication of diabetes. We accomplished a proof-of-concept experiment proving that oral supplementation of PPi was able to inhibit DM-associated calcification. The successful outcome of this experiment provides the preclinical basis of a mechanism-based first-in-class therapy for DM-associated calcification and other disorders of abnormal ectopic calcification (Small scale prototype).
Intellectual property:
Granted Patent EP 3512530 B1 “Oral Pyrophosphate for Use in Reducing Tissue Calcification” and US11504395B2 (same title). The invention was developed in research collaboration of the Research Center for Natural Sciences (RCNS) and the Netherland Cancer Institute (NKI) in 2016. The contributions to the invention of the respective employees of the two institutions are 85% (RCNS) and 15% (NKI). The patent was filed by NKI in September 2016, who undertakes to create the conditions for exploitation mainly through licensing agreements.